
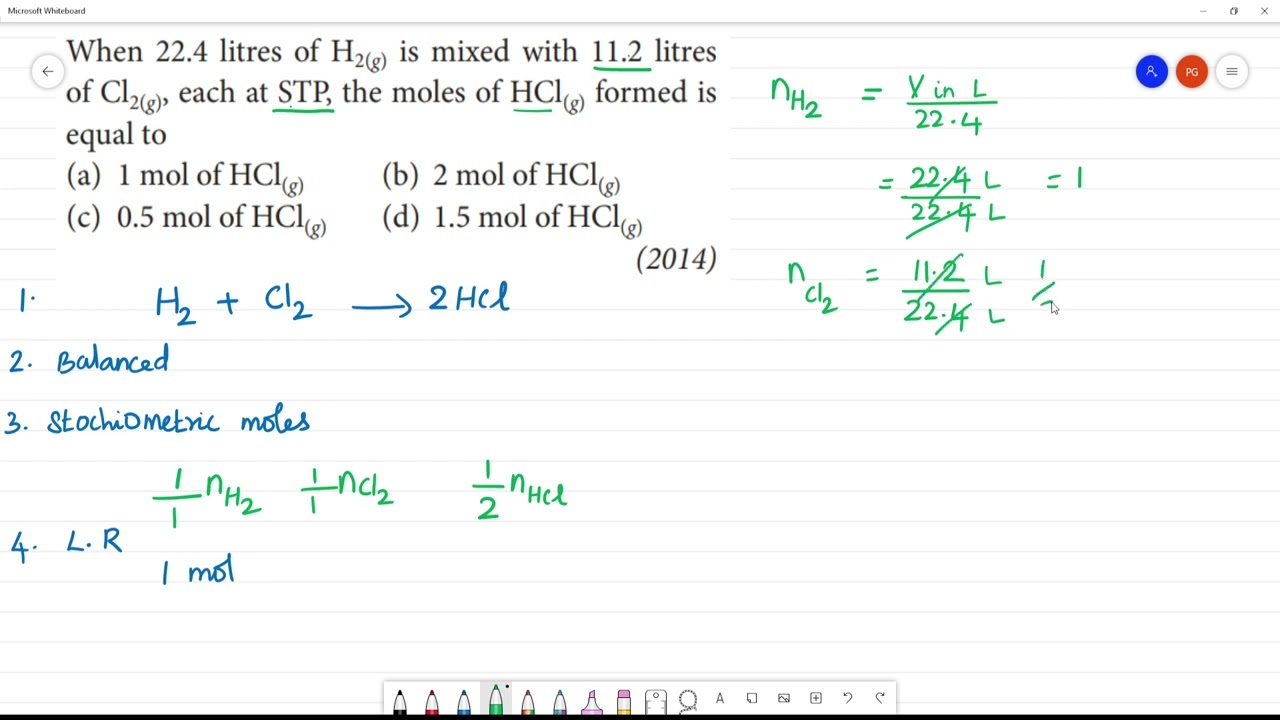
WebNCERT | Chemistry | Class 11| SnapsolveWhen 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2 (g), each at S.T.P, the moles of HCl (g) formed is equal to... WebWhen 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2(g), each at S.T.P, the moles of HCl(g) formed is equal to :-. <!--td {border: 1px solid #ccc;}br {mso-data-placement:same.
When 22 4 Litres Of H2, When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2 (g), each at S.T.P, the...| SnapSolve, 6.02 MB, 04:23, 763, SnapSolve, 2021-05-19T09:53:28.000000Z, 19, 45. M 37. When 22.4 litres of H2(8) is m... - Inorganic Chemistry, questions-in.kunduz.com, 2107 x 512, jpeg, , 20, when-22-4-litres-of-h2, KAMPION
WebSommaire. 1 Rappeler la formule liant la quantité de matière au volume des gaz 2 Repérer la quantité de matière 3 Repérer le volume molaire 4 Effectuer l'application numérique.. Web1 mol of any gas at STP occupies 22.4L (Ideal Gas Law) 4.0L H2 is therefore 4/22.4 = 0.1786mol. So we need 0.1786mol of F2, since the formula for hydrogen fluoride is HF.. WebOct 02, 2020. Related When 22.4 litres of H2 is mixed with 11.2 litres of Cl2,each at S.T.P, the moles of HCL(g) formed is equal to? 1 mole of H2 will react with 1. Web22.4L volume at STP is occupied by H 2 = 1mol. Since, C l2 possesses minimum number of moles, thus it is the limiting reagent. As per equation, 1 mole of C l2 = 2 moles of H Cl. ∴. WebSince 22.4 litres of a gas occupies 1 mole. Hence, moles of 12 L of H 2 will be = 22.412 =0.54 moles. Similarly moles of 11.2 L Cl 2 gas = 22.411.2=0.5 moles. Cl 2 is the limiting. WebMay 25, 2014. The Molar Volume of an ideal gas at STP, which we define to be 0∘C and 1 atm arbitrarily (because we're old-fashioned and stuck in 1982) is 22.411. WebClick here👆to get an answer to your question ️ If 0.224 litre of H2 gas is formed at the cathode, how much O2 gas is formed at the anode under identical condition? Solve Study.
Currently - When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2 (g), each at S.T.P, the...| SnapSolve New
Watch When 22.4 litres of H2(g) is mixed with 11.2 litresof Cl2(g), each at STP, the moles of HCl(g) form Latest
Other descriptions of When 22 4 Litres Of H2 Next
NCERT | Chemistry | Class 11| Snapsolve
When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2 (g), each at S.T.P, the moles of HCl (g) formed is equal to :
(A) 0.5 mol of HCl (g)
(B) 1.5 mol of HCl (q)
(C) 1.0 mol of HCl (g)
(D) 2·0 mol of HCl (g)
snapsolve.com/class10/math/ncert-1108549627
SnapSolve is a doubt-solving and learning app that provides students from Class 6 to 12 with free solutions for doubts. You can take pictures of homework or book questions and get answers and solutions in just one second! We also offer live online tutoring in which you get solutions from tutors from IITs and other top educational institutions.
Download SnapSolve NOW: z.snapsolve.com/QBDsSpbwv7
🌐 Website: snapsolve.com/
Subscribe Our YouTube Channels:
✿ SnapSolve: youtube.com/channel/UCT0B3OIJxDXz7B-yRp58bQQ
✿ SnapSolve Live Class: youtube.com/channel/UCWGuJstzjNAYPf4Vm3DdUoA
----------------------------------------------------------------------------------------------------
Watch More Videos Solutions:
📌CBSE NCERT 9TH Full Course: youtube.com/channel/UCT0B3OIJxDXz7B-yRp58bQQ/playlists?view=50&sort=dd&shelf_id=4
📌CBSE NCERT 10TH Full Course: youtube.com/channel/UCT0B3OIJxDXz7B-yRp58bQQ/playlists?view=50&sort=dd&shelf_id=2
📌CBSE NCERT 11TH Full Course: youtube.com/channel/UCT0B3OIJxDXz7B-yRp58bQQ/playlists?view=50&sort=dd&shelf_id=5
📌CBSE NCERT 12TH Full Course: youtube.com/channel/UCT0B3OIJxDXz7B-yRp58bQQ/playlists?view=50&sort=dd&shelf_id=1
📌 JEE Physics Full Speed Crash Course: youtube.com/watch?v=5d9DX9J_KSA&list=PLQVkKs0-Ll8weE609a_imb94RwzJ_tpQw
📌JEE Chemistry Full Speed Crash Course: youtube.com/watch?v=aouhLLU3-VA&list=PLQVkKs0-Ll8xq7tlgGjbHIp9gIyLpfXyv
📌JEE Maths Full Speed Crash Course: youtube.com/watch?v=tmLAHgdiC0s&list=PLQVkKs0-Ll8wFzgxkV53VnR1KI5IFQ9dA
📌Maths Tricks: youtube.com/playlist?list=PLQVkKs0-Ll8yo7oP-4tiuecriZyAU4TZA
📌SnapSolve Preparation League: youtube.com/channel/UCT0B3OIJxDXz7B-yRp58bQQ/playlists?view=50&sort=dd&shelf_id=7
📌Doubt Videos: youtube.com/channel/UCT0B3OIJxDXz7B-yRp58bQQ/playlists?view=50&sort=dd&shelf_id=8
----------------------------------------------------------------------------------------------------
Follow Us on These Platforms:
🔔 Facebook: facebook.com/snapsolveofficial/
🔔 Instagram: instagram.com/snapsolve_official?igshid=9utnlgjhqk5
🔔 Telegram: t.me/snapsolve
🔔 LinkedIn: linkedin.com/company/snapsolve
Our Telegram Pages:
🔔 Snapsolve Official - Study channel for Grade 6-12: t.me/snapsolve
🔔 Snapsolve VIP study group: t.me/snapsolve_group
🔔 Snapsolve Bonus Group: t.me/joinchat/TkEhPjz6jJSUI971
🔔 Official SnapSolve Class 6-8: t.me/StudyWithSnapsolve
🔔 Snapsolve Live Quiz: t.me/SSLiveQuiz
🔔 Official SnapSolve Class 11&12: t.me/joinchat/VvjHS1QCASnDaHml
🔔 Official SnapSolve Class 9&10: t.me/joinchat/VkzuryfAmEbQa4vB
Contact Us:
👉 Have Any Query? Ask Us.
📧 Email: feedback@snapsolve.com
About 45. M 37. When 22.4 litres of H2(8) is m... - Inorganic Chemistry

Must see When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2(g) , each at Latest

How many moles of HCl formed when 22.4 liters of H2 gas is mixed with going viral
ntTwo moles of N2 and two moles of H2 are taken in a closed vessel of 5 Latest
Let's see 4.0 g of a gas occupies 22.4 litres at NTP. The spec... - Physics

Topics Solved what mass of Al must react to give 22.4 L of H2 at | Chegg.com updated
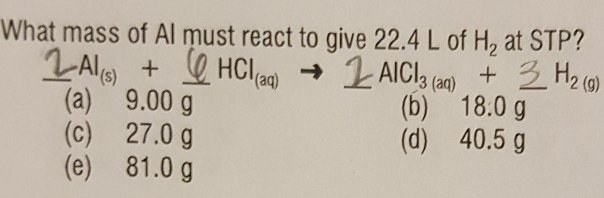
Must see ar 26. 4.0 g of a gas occupies 22.4 litres at NTP. T... - Physics update

Viral When 22.4 litres of H2 is mixed with 11.2 litres of Cl2 then what are
Viral (4) 22.4 litres of the solution LS0022 22... - Physical Chemistry going viral

Subject One gram mole of a gas at NTP occupies 22.4 litres as volume. This fact trending


0 komentar:
Posting Komentar