
WebpH of a saturated solution of `Ca (OH)_ (2)` is 9. the solubility product ` (K_ (sp))` of `Ca (OH)_ (2)` is Previous Year Question for NEET by Doubtnut. WebQ. pH of a saturated solution of Ca(OH)2 is 9. The solubility product (Ksp) of Ca(OH)2 is: Q. pH of a saturated solution of Ca(OH)2 is 9. The solubility product (Ksp) of Ca(OH)2 is: Q..
Ph Of Saturated Solution Of Ca Oh 2 Is 9, pH of a saturated solution of `Ca(OH)_(2)` is 9. the solubility product `(K_(sp))` of `Ca(OH)_(2)`, 4.9 MB, 03:34, 7,115, CBSE Class 11 & 12, 2020-02-04T07:32:31.000000Z, 19, 74. pH of a saturated solution of Ca(OH)2... - Physical Chemistry, questions-in.kunduz.com, 3470 x 512, jpeg, , 20, ph-of-saturated-solution-of-ca-oh-2-is-9, KAMPION
WebCalculating the pH of a solution of Ca (OH)2. acid-base ph. 20,243. You did not account for the fact that $\ce {Ca (OH)2}$ is partly precipitated. Firstly, to find out the. WebCalculating the pH of a solution of Ca (OH)2. Ask Question. Asked 9 years, 4 months ago. Modified 9 years, 2 months ago. Viewed 21k times. 1. A solution prepared by dissolving. WebpH of a saturated solution of Ca(OH)2 is 9. The Solubility product (K) of Ca(OH)2 (a) 0.5 x 10-15 ... 10 (c) 0.125 x 10-15 (d) 0.5 x 10-10 The Solubility product (K). WebThe solution is acidic if its pH is less than 7. If the pH is higher, the solution is basic (also referred to as alkaline). Solutions with a pH that is equal to 7 are neutral.. WebAt last, added sugar remains as solid on the bottom of the container, then the solution is said to be saturated. Given saturated solution is $Ca{{(OH)}_{2}}$ The pH. WebExplanation: ...we are given that pH = 9.20 ...and thus pOH = 14.0 −9.20 = 4.80 .. And so [H O−] = 10−4.80 = 1.58 × 10−5 ⋅ mol ⋅ L−1 .. And (ii), we write a reaction. WebKsp = [Ca2+][H O−]2. And so if we represent the solubility of calcium hydroxide as S, then, Ksp = (S)(2S)2 = 4S3. ...and S = 3√ 5.5 × 10−6 4 = 1.11 × 10−2 ⋅. WebThe solubility product (Ksp) of Ca(OH)2 is: Q. pH of a saturated solution of Ba(OH)2 is 12. The value of solubility product (Ksp) of Ba(OH)2 is : Q. The solubility product (Ksp) of.
Discussion pH of a saturated solution of `Ca(OH)_(2)` is 9. the solubility product `(K_(sp))` of `Ca(OH)_(2)`
Currently - ph of a saturated solution of Ca(OH)2 is 9. The solubility product(Ksp ) of Ca(OH)2 is Latest
Explanation of Ph Of Saturated Solution Of Ca Oh 2 Is 9 latest
pH of a saturated solution of `Ca(OH)_(2)` is 9. the solubility product `(K_(sp))` of `Ca(OH)_(2)` is neet 2020 study plan,neet 2020 preparation,neet 2020 syllabus,neet 2020 cut off for government colleges,neet 2020 latest news,neet exam 2020,neet exam 2020 details,neet exam preparation,neet exam,neet exam questions in hindi,neet 2020,neet important questions 2020,neet important chapters 2020,neet important questions 2020 chemistry,neet important questions 2019 chemistry,neet sample paper 2020,neet mock test online free,neet mock test
74. pH of a saturated solution of Ca(OH)2... - Physical Chemistry trending

Look pH of a saturated solution of Ca(OH)(2) is 9. the solubility product (K Latest

Must see pH of a saturated solution of `Ca(OH)_(2)` is 9. the solubility product updated

Latest The pH of a saturated solution of Fe(OH)_(2) is 8.67. What is the K_(sp

Look Ph of a saturated solution of ca(oh)2 is 9. The solubility product (ksp popular

Solved The pH of a saturated solution of Mn(OH)_2 is 9.86. | Chegg.com

Currently - OneClass: Students test a saturated solution of Bs(OH)2 and find its pH trending

pH of a saturated solution of Ca(OH)2 is 9. The solubility product (Ksp

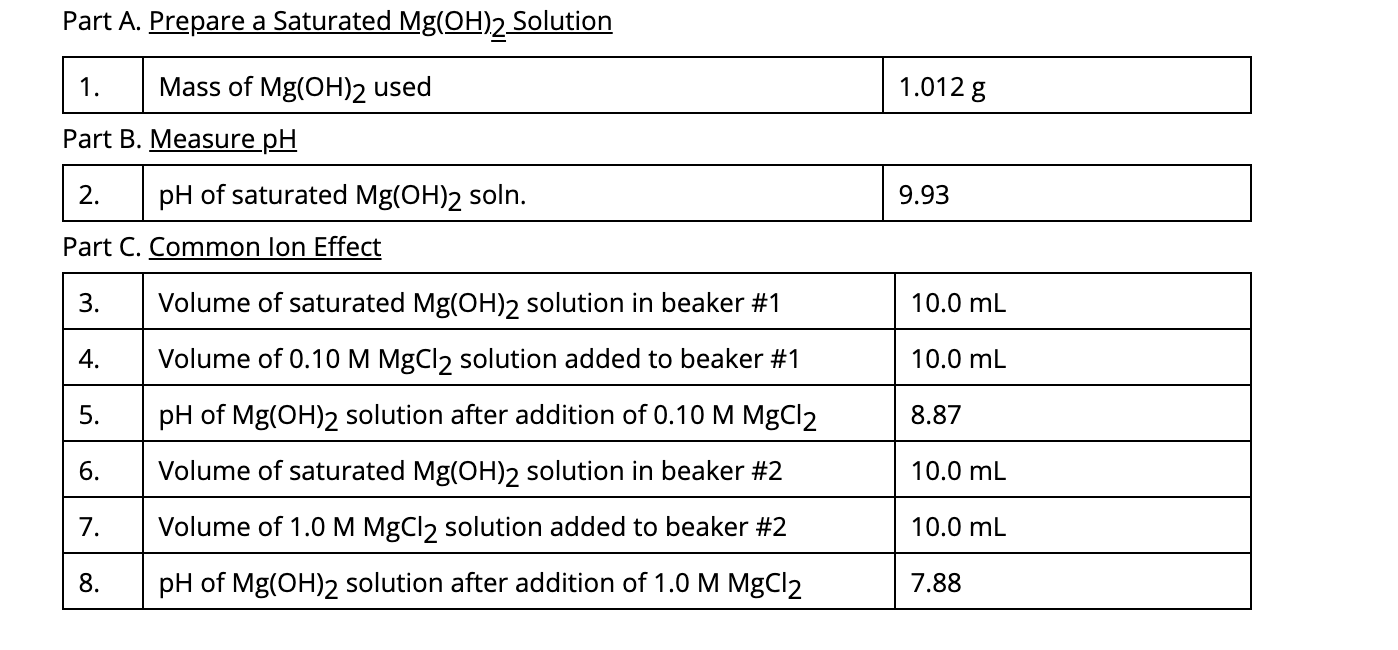
Reviews Solved Part A. Prepare a Saturated Mg(OH)2 Solution 1.012 g | Chegg.com trending
Solved What is the pH of a saturated solution of Zn(OH)_2? | Chegg.com



0 komentar:
Posting Komentar