
WebThe energy required to break one mole of `Cl-Cl` bonds in `Cl_2` is `242 kJ mol^-1`. The longest wavelength of light capable of breaking a since `Cl-Cl` bond is ...more. ...more. WebEnergy required to break 1 mole of Cl-Cl bonds = 242 kJ. Energy required to break 1 Cl-Cl bond = 6.023×10 23242×10 3 J. If the wavelength of light is largest, then it would. WebBond dissociation energy is the amount of energy required to break a chemical bond between A and B A − B . The bond dissociation energy depends on the.
The Energy Required To Break One Mole Of Cl Cl, The energy required to break one mole of `Cl-Cl` bonds in `Cl_2` is `242 kJ mol^-1`. The longest, 4.21 MB, 03:04, 2,080, Doubtnut, 2020-01-19T06:49:24.000000Z, 19, The energy required to break one mole of Cl-Cl bonds in Cl2 is 242 kJ, www.doubtnut.com, 1200 x 677, png, , 20, the-energy-required-to-break-one-mole-of-cl-cl, KAMPION
WebStep 1: Bond energy : It is also known as the bond enthalpy. It is a measure of the bond strength of a chemical bond, It is also the amount of energy required to break one mole of. WebThe energy required to break one mole of Cl − Cl bonds in C l 2 is 242 kJ m o l − 1. The longest wavelength of light capable of breaking a single Cl − Cl bond is ( C = 3 × 1 0 8 m. WebFor calculation of longest wavelength of light that can break Cl-Cl bond can be found by using Formula E = Nhc/λwhere h= planck's constant, c = speed of light, λ = wavelength. WebThe energy required to break one mole of Cl Cl bonds in Cl2 is 242 kJ mol 1. The longest wavelength of light capable of breaking a single Cl Cl bond is: c=3× 108 m/s and. WebA bond energy is the amount of energy. needed to break one mole. of a particular covalent bond. Different bonds have different bond energies. These are given when they are. WebThe bond energy also known as the bond enthalpy or the bond strength is the amount of energy that is required to break a bond or a mole of a molecule into its.
Articles The energy required to break one mole of `Cl-Cl` bonds in `Cl_2` is `242 kJ mol^-1`. The longest updated
New Atomic structure 10: The energy required to break one mole of 𝐶𝑙−𝐶𝑙 viral
Explanation The Energy Required To Break One Mole Of Cl Cl from the discussion earlier
The energy required to break one mole of `Cl-Cl` bonds in `Cl_2` is `242 kJ mol^-1`. The longest wavelength of light capable of breaking a since `Cl-Cl` bond is
About The energy required to break one mole of Cl-Cl bonds in Cl2 is 242 kJ New

Look The energy required to break one mole of Cl-Cl bonds in Cl2 is 242 kJ
The energy required to break one mole of Cl – Cl bonds in Cl2 is 242 kJ

New Solved The energy required to break one mole of | Chegg.com more

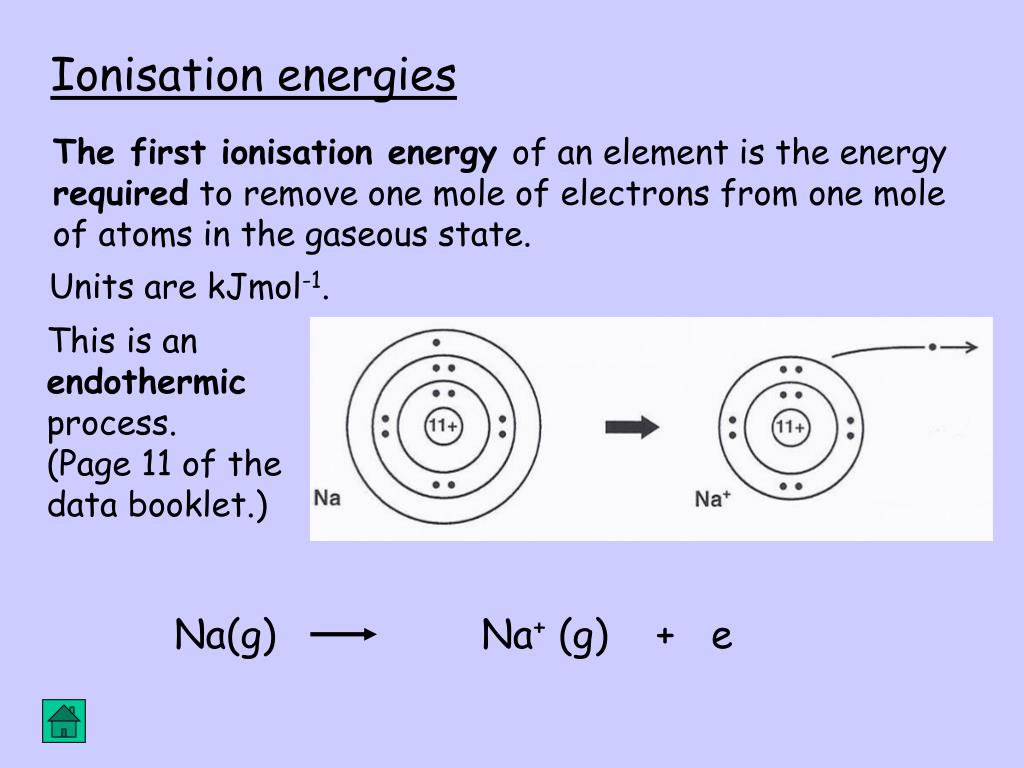
News PPT - Section 2 PowerPoint Presentation, free download - ID:5963355 New
About The energy required to break one mole of Cl - Cl bonds in Cl2 is 242kJ more

News Calculate the wavelength of light required to break the bond between

Let's see Calculate the wavelength of light required to break the bond between updated

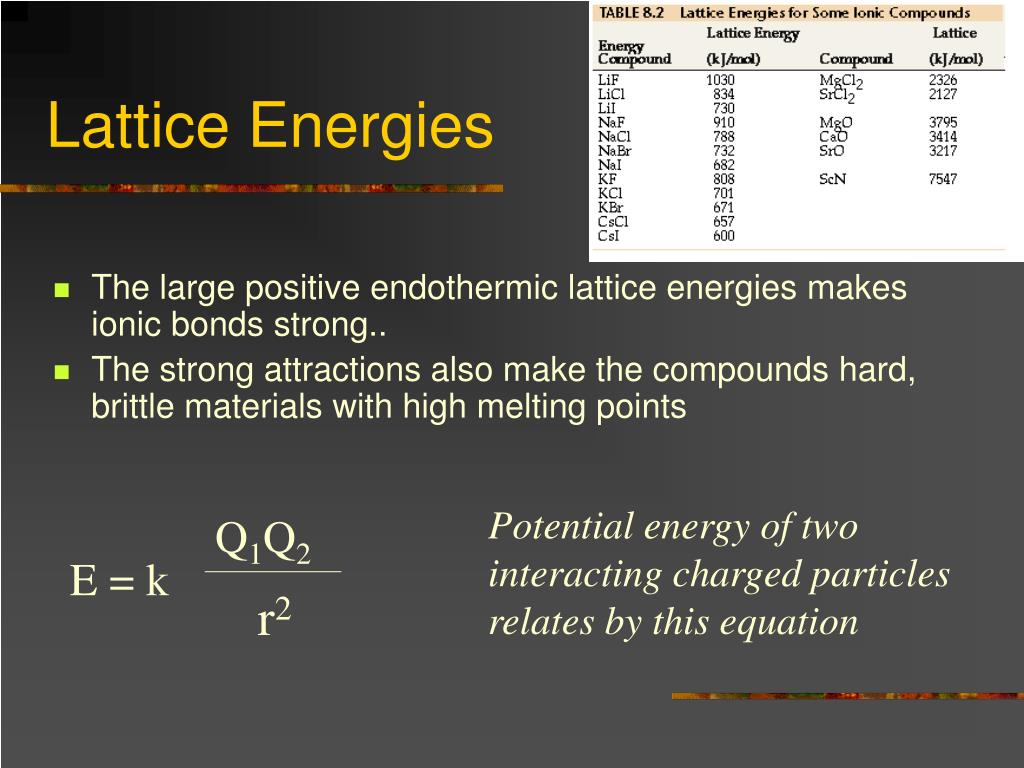
Articles PPT - Chemistry 100 Chapter 8 PowerPoint Presentation, free download viral

Articles PPT - Advanced Chemistry PowerPoint Presentation, free download - ID more


0 komentar:
Posting Komentar